Abstract
Introduction: In complex treatment programs such as allogeneic hematopoietic stem cell transplant (alloHCT), clear and effective communication between patients and providers is critical. Language and literacy skills are known to impact health outcomes, but the effect of primary language on allogeneic HCT outcomes is unclear. We hypothesized that differences in primary language (English versus non-English) would be associated with differences in outcome among recipients of allogeneic HCT.
Methods: This was a retrospective analysis of patients who underwent allogeneic HCT at a single center between 2010 and 2020. Patients were included regardless of graft source or conditioning regimen. Primary language preference was based on patient self-report documented in electronic health records. The relationship between primary language, and overall survival (OS) and progression-free survival (PFS) were univariably analyzed via Kaplan-Meier and log-rank tests. To adjust for age and graft source, a Cox proportional hazards model was used to quantify the hazard of mortality for non-English primary language versus English primary language transplant recipients.
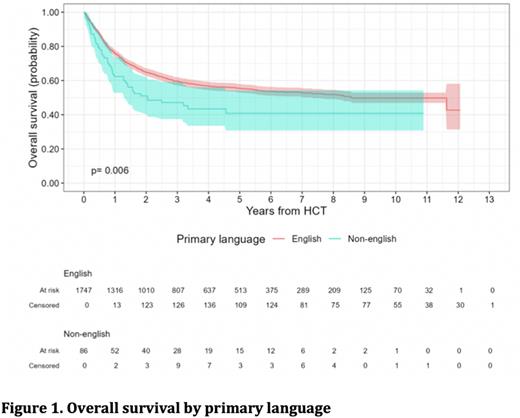
Results: A total of 1833 patients were included in the analysis. Median age in our cohort was 57 years (range: 18-79) with 41% females (755) and 59% males (1078). There were 86 non-English primary language patients and 1747 English primary language patients. A total of 18 languages were included in the non-English primary language cohort: Spanish, Mandarin, Arabic, Cantonese, Russian, Korean, Portuguese, Sign Language, Albanian, Haitian Creole, Hindi, Italian, Lao, Persian, Hebrew, Romanian, Old Norse, and Twi. Leukemia was the most common indication for transplant with 63% of non-English primary language speaking patients and 54% of primary language English speaking patients. OS for non-English primary language speaking patients was significantly lower than for English primary language speaking patients at 62% (95% confidence interval (CI) 53-74%) versus 76% (95%CI 74-78%) at 1 year post alloHCT(p=0.006). After adjusting for age and graft source, non-English primary language speaking patients remained at higher risk of death than their counterparts (hazard ratio: 1.51, 95% CI 1.13-2.03). Median PFS was longer for English primary language patients than for non-English primary language patient: 3.5 years (95%CI 2.7, 5.6) vs 0.78 years (95%CI 0.59, 2.0) respectively (p<0.001).
Conclusion: In this single-center analysis, non-English primary language was significantly associated with poorer OS and PFS for allogeneic HCT recipients. Larger studies are needed to confirm these findings and to better understand the underlying factors contributing to differences in survival. Most importantly, this highlights the urgent need for practical interventions to improve outcomes among traditionally marginalized populations.
Disclosures
Perales:Abbvie: Honoraria; Astellas: Honoraria; Celgene: Honoraria; Karyopharm: Honoraria; MorphoSys: Consultancy, Honoraria; Takeda: Honoraria; Medigene: Consultancy; Servier: Consultancy; Bellicum: Honoraria; DSMB: Other; Orca Bio: Consultancy; Sellas Life Sciences: Consultancy; Cidara Therapeutics: Consultancy; Vor Biopharma: Honoraria; VectivBio AG: Honoraria; Nektar Therapeutics: Consultancy, Honoraria; Omeros: Consultancy; Novartis: Honoraria; Miltenyi Biotec: Consultancy, Honoraria; Merck: Consultancy; Kite, a Gilead Company: Honoraria, Research Funding; Incyte: Honoraria, Research Funding; Bristol-Mysers Squibb: Honoraria. Giralt:Miltenyi: Research Funding; Spectrum Pharma: Consultancy; Novartis: Consultancy; Janssen: Consultancy; Omeros: Research Funding; Takeda: Consultancy, Research Funding; Johnson & Johnson: Consultancy, Research Funding; Kite: Consultancy; Jazz Pharmaceutical: Consultancy; Amgen: Consultancy, Research Funding; Actinuum: Consultancy, Research Funding; Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal